Title 21, Code of Federal Regulations, Part 110.110 allows the Food and Drug Administration (FDA) to establish maximum levels of natural or unavoidable defects in foods for human use that present no health hazard. These “Food Defect Action Levels” are set on this premise–that they pose no inherent hazard to health.
Poor manufacturing practices may result in enforcement action without regard to the “action level:. Likewise, the mixing or blending of food with a defect at or above the current defect action level with another lot of the same or another food is not permitted. That practice renders the final food unlawful regardless of the defect level of the finished food.
The FDA set these action levels because it is economically impractical to grow, harvest, or process raw products that are totally free of non-hazardous, naturally occurring, unavoidable defects. Products harmful to consumers are subject to regulatory action whether or not they exceed the action levels.
It is incorrect to assume that because the FDA has an established defect action level for a food commodity, the food manufacturer need only stay just below that level. The defect levels do not represent an average of the defects that occur in any of the products–the averages are actually much lower. **The levels represent limits at which FDA will regard the food product “adulterated”; and subject to enforcement action under Section 402(a)(3) of the Food, Drug, and Cosmetics Act. **
As technology improves, the FDA may review and change defect action levels on this list. Also, products may be added to the list. The FDA publishes these revisions as Notices in the Federal Register. It is the responsibility of the user of this booklet to stay current with any changes to this list.
If there is no defect action level for a product, or when findings show levels or types of defects that do not appear to fit the action level criteria, FDA evaluates the samples and decides on a case-by-case basis. In this procedure, FDA's technical and regulatory experts in filth and extraneous materials use a variety of criteria, often in combination, in determining the significance and regulatory impact of the findings.
The criteria considered is based on the reported findings (e.g., lengths of hairs, sizes of insect fragments, distribution of filth in the sample, and combinations of filth types found). Moreover, FDA interprets the findings considering available scientific information (e.g., ecology of animal species represented) and the knowledge of how a product is grown, harvested, and processed.
It is FDA's position that pesticides are not the alternative to preventing food defects. The use of chemical substances to control insects, rodents and other natural contaminants has little, if any impact on natural and unavoidable defects in foods. The primary use of pesticides in the field is to protect food plants from being ravaged by destructive plant pests (leaf feeders, stem borers, etc.).
A secondary use of pesticides is for cosmetic purposes–to prevent some food products from becoming so severely damaged by pests that it becomes unfit to eat.
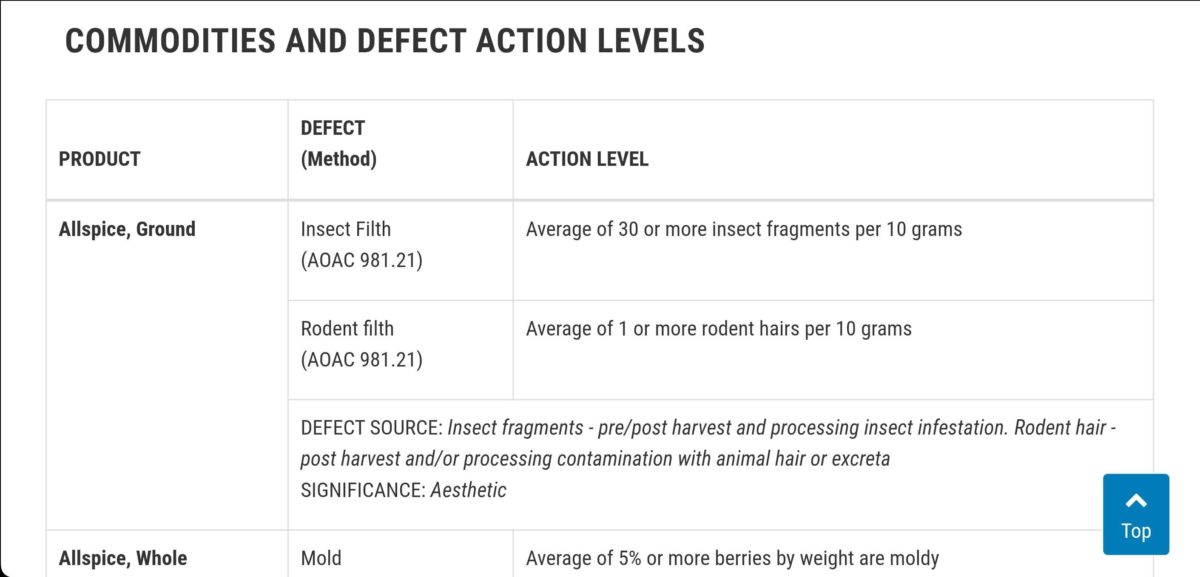
This edition of The Food Defect Action Level includes the source of each defect and the significance of it (i.e., how the defect affects the food). Food processors may find this information helpful as a quality control tool in their operation.
Food commodities (Product) are listed alphabetically. Each listing indicates the analytical methodology (Defect Method) used, as well as the parameters for the defect (Defect Action Level).
The Macroanalytical Procedures Manual (MPM) is out of print. However, it is available on the web at Macroanalytical Procedures Manual.
Snowpiercer wasn't supposed to be real life, but ok